Aaron Siri and the V-Safe Data:
The following is partly gleaned from an Aaron Siri interview with Shannon Joy. The rest comes directly from Aaron Siri via his Substack as well as some other news sites with recent updates. You will recall that Aaron Siri is the attorney working with ICAN (the Informed Consent Action Network). It is because of his work that the clinical trial data was ‘pried from the cold dead hands’ of Pfizer (and the FDA), albeit at a snail's pace. And Siri has worked on getting the v-safe data as well.
First a Recap of the V-safe APP History:
First this quick recap, to bring you back up to speed. In December 2020 the mRNA shot was released to much fanfare. V-safe, the APP, was rolled out at that time, to be used for real-time tracking of adverse events, specifically for this shot. The APP may be called an ‘active surveillance system’ as opposed to VAERS which is delayed and ‘passive’ as it is only activated if an injury is suspected, in retrospect. Importantly, to be posted to VAERS, a medical intermediary almost always is needed to agree that the symptoms are shot-related … and that almost never happens. The reasons for this are numerous and include - doctors have been told that the ‘vaccines’ are safe and so what appear to be adverse reactions are really just coincidences & doctors are busy and it is time-consuming to place an entry, so mostly they don’t bother. And still the VAERS database exploded with reports of adverse events. Some estimate that one needs to multiply those by a factor of 40 or so to get to a truer number.
In the instance of v-safe however, the shot recipients themselves (some 10 million or so used the APP) were to fill out a report daily for seven days, then weekly for 5 more weeks, then at 3, 6, and 12 months. So data was gathered from all comers, not just the injured. And those with no issues also filled it out … not just those with potential side effects. V-safe was rolled out from Dec 2020 to May 2021, prior to any mandates for getting the shot. Thus, if anything, users at that point in time, largely self selected for being the ones most eager to get the shot; not skeptics. These were generally the “vaccine” enthusiasts and celebrants. It started with elderly and healthcare workers and those who were so anxious to get the shot, that they cut into line.
So here we have a subset of those least likely to complain. After about a year’s worth of data was collected, ICAN requested the data. The CDC had said (prior to the rollout) that they would be completely transparent and release the data to the public. Strangely, they had a change of heart and they did not release the promised data. Weird, right?!
So a FOIA was submitted and the CDC finally (after a lengthy legal battle) was compelled to release the data. Even with that, they complained to the court that they didn’t have enough money and help to get this done (probably too many billions going to foreign wars). They essentially refused to release what they previously said that they would voluntarily (expecting wonderful results perhaps?!). The legal process took roughly a year and a half. Ultimately the CDC ‘capitulated’ and sent out the ‘checkmark data.’ But they withheld the ‘free text data.’ More on that …
Free-text Data Fields:
It took another lawsuit to free up the free-text entries..
The ‘check boxes’ in the APP had about ten options that a shot recipient could utilize - all were essentially the ‘normal’ reactions one might expect after an immunization. A little pain, a little swelling - all of these are ‘normal’ and even ‘good,’ we are told … shows the shots are working, don’t you know. It’s what we want to see. Sure enough, some 60-80% reported symptoms and the CDC said ‘great.’ All is well. The shots are working.
But what about those who had additional symptoms not in the pre-configured checklist of ‘normal’ reactions? What were they supposed to do? Their only option was to write in their experiences - in a ‘free-text’ data field (maximum 250 characters). The CDC, however, never released the ‘free text’ write-in adverse events (that were clearly being discouraged from being documented as there was no easy way to enter these). Now we know that there were some 7.8 million of these free text entries..
One of the heads at the CDC said ‘we can’t assess safety with this’ - referring to the checklist data … it’s essentially useless. There’s no point even gathering this data. Myocarditis, chest pain, clots, and so on - are not even on the checklist. The important data was hidden from view.
We know that the CDC had developed a list of what it called Adverse Events of Special Interest (AESIs). The list included myocarditis, pericarditis, transverse myelitis, blood clots and so on. But none of these ended up being included on v-safe’s checklist, despite the build protocol actually calling for building the APP with these AESIs included. Somebody apparently made the decision specifically to exclude them.
Here is a listing of some of the AESIs that were to have been included in the APP’s build. Note that the CDC understood that there would be limitations on capture of ‘death’ as an adverse event … since the dead stop using their APPs.
Why, do you suppose, that the CDC’s v-safe check list included things like headache, nausea, and generalized aches and pains … but did not include things like seizures, paralysis, leg numbness, fainting, chest pain and the like? Could it be that they wanted their ‘active surveillance system’ to under-count and under-estimate any potentially bad adverse reactions? Motives are hard to assess. However, IF I wanted to minimize the appearance of adverse events, that’s exactly how I would have built the APP. Just sayin’ …
It almost seems like the CDC was manipulating v-safe results towards a predetermined finding … of safety. Or perhaps they are incompetent. You pick whichever explanation you’d like. As you pick, do bear in mind that the CDC has hired some of the nation’s sharpest minds. mRNA vaccines, by the way, had been tried in the past and always unsuccessfully. Those prior experiences likely drove the list of adverse events of special interest … that suddenly the CDC seemed disinterested in.
The CDC created a short video for APP users, on how to use v-safe. In addition to the check boxes mentioned, I should note that there was also a place to record, if perchance you did have a reaction, whether that reaction led to an “adverse health impact.” This section contained three choices: mild, moderate or severe impact. I will give some of those results in the next section.
Remember that while the CDC was fighting for their inalienable right to hide the data from the public that paid for it, they were parroting how safe and effective the “vaccines” were. So safe, in fact, that they didn’t want you to see the results. You wouldn’t know how to interpret them and heaven forbid you might come to a conclusion that the shots were unsafe.
Early Data:
But even before the “free text data” was ordered released, the initial lawsuits came up with some pretty damning statistics - just based on the checkbox and ‘Adverse Health Impact’ data.
The “Adverse Health Impacts” section showed that in week 1 post injection, a whopping 782,913 users (out of the 10,108,273) reported requiring medical care. During the first 6 weeks of follow up, 7.7% of users needed medical help. Over 70% of those went to an emergency department, or urgent care or were hospitalized. Another 25% reported being unable to continue some of their regular activities. So that’s over 32% who needed help or were out of commission to some degree.
How is it that our agencies claimed that if you were in the military and didn’t get the shot it would hurt military readiness? Seems that getting the shot would be worse for military readiness.
The CDC, as a governmental agency, sets policies. They are not in fact a healthcare institution. Thus their premier publication, MMWR (Morbidity and Mortality Weekly Reports), publishes only studies that conform to the policy. Thus they chose to publish only select data that reflected less poorly on the shots and ignored anything that might make them look bad. Anything to support the policy that they had already predetermined. I used to trust the MMWR and in fact was a subscriber for many years. And likely - most of what was contained therein, was trustworthy. I now wonder how much was not.
Reports in MMWR, that is from the CDC, focused on the least problematic data such as the week post-shot that had the least number of reported issues. Just like with our ‘trusted news,’ sometimes it’s not what they say that’s the problem, but rather what they ignore. Still, between those that sought medical help and those that could not carry on their daily activities often including going to work, the total was roughly 32% of shot recipients. That is huge.
The CDC is a ‘captive’ agency - impenetrable and inscrutable. These same folks have been in the business of approving vaccines for us all (and especially our kids) for many years. Since there is no legal recourse to sue if they turn out to be harmful, there is nothing putting the brakes on reckless recommendations. Thus we have seen an explosion of likely dangerous and frequently completely unnecessary vaccines. Clinical trial periods have grown shorter and shorter and scrutiny for safety and efficacy has been truncated. We are somewhere approaching 90 vaccine doses before age 18 if you include the new prenatal assault on kids' immune systems (that is giving newborns immunity to a disease by vaccinating the mother during pregnancy).
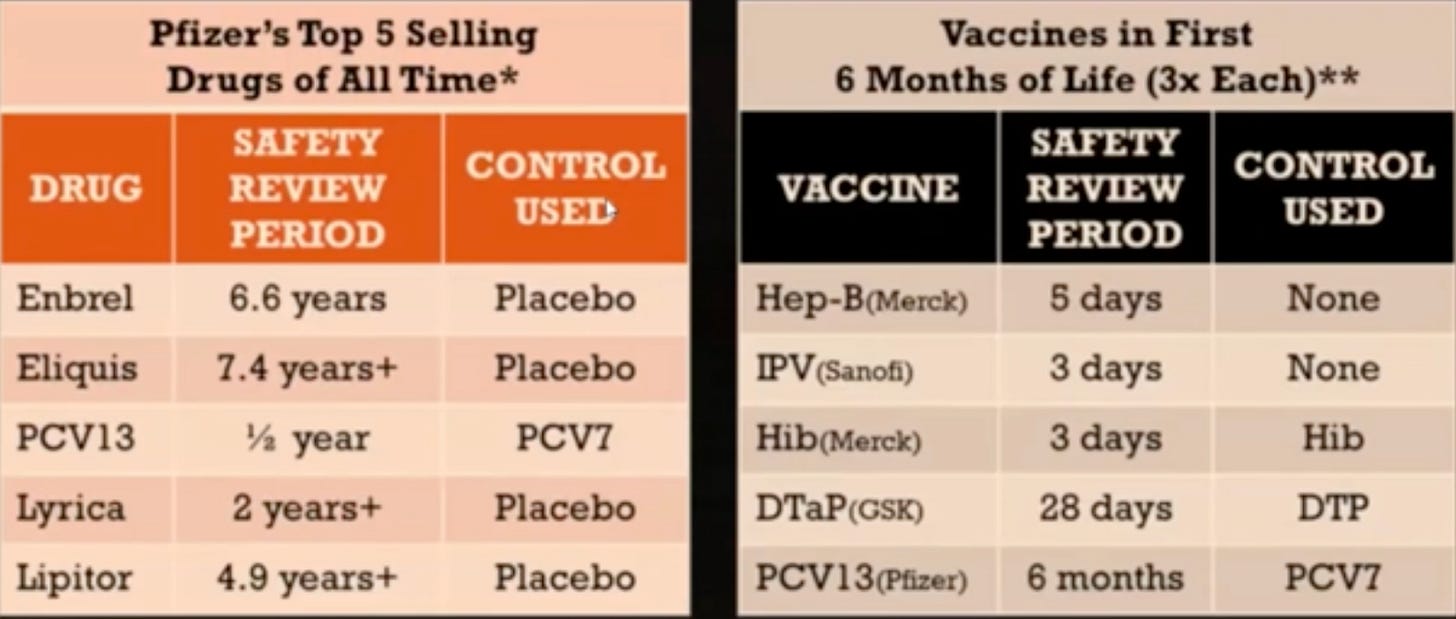
One must wonder why most drugs are tested against an actual placebo whereas vaccines are tested against other vaccines for safety. The apparent answer is lack of liability and the ‘need’ to show that the shots are safe (compared to placebo wouldn’t work out, so they compare to another not-so-safe shot).
So, you might ask, what is the threshold of adverse events that would prompt the CDC to discontinue usage of a vaccine product? Well, it seems that there is none. They decide based on whatever data they choose to include in their discussions. And therein lies the rub. They pick and choose in a rather subjective manner.
The Court’s Decision:
Back to our update on the “free-text” data. The court has ordered the release of the 7.8 million data entries over the next twelve months (from its roughly 10 million users). The first ‘dump’ just occurred on 2/15. Here are some quotes from the court rulings, which I found to be well written.
“... the federal government collaborated and cooperated with foreign governments and non-governmental humanitarian organizations, private companies, and the media to enable and incentivize widespread vaccination… The government promoted vaccination - directly through mandates or indirectly through policies, privileges, and messaging campaigns. Many employers required vaccination … Societal reality hinged on vaccination status - from school attendance to family vacations. By early 2023, more than 5.5 billion people (about 72.3 percent of the world population) had received a dose of a COVID-19 vaccine, including more than 270 million Americans. Defendants have consistently asserted that “COVID-19 vaccines are safe and effective,” recommends everyone ages 6 months and older get an updated COVID-19 vaccine,” and added the COVID-19 vaccine to standard Child and Adolescent Immunization Schedule … It is with this background that Plaintiff aims to further the ideals pledged by the Biden-Harris administration: to “Promote trust, transparency, common purpose, and accountability in our government” by making available for public access - and particularly for independent scientific and medical research - all of the relevant health data collected through the V-safe program. …
The simple reason Defendants denied Plaintiff’s production request is the 7.8 million free-text response entries are allegedly too numerous for the agency’s limited resources … While the burden to produce the requested free-text responses may be heavy, this Court does not find that it is unreasonable … “
The court decision further notes that the CDC has only published some results relating to the first week of v-safe results, with a single study only looking as far out as 2 weeks. The CDC admits to this but they opined that the reason was that it was “the time period that some scientists have chosen to use in their research studies.” Why they would “choose” to only look at this brief period when they have vastly more data, is open to speculation. But I cannot think of any reason beyond their wanting to only look at a period that they could use to promote their product while ignoring any adverse study period. I’m open to a non-conspiratorial theory. I just can’t think of one offhand.
Is this even credible? It’s as if they are saying: ‘We collected all of this data because we needed to take a close look at what is happening in a shot being foisted upon the entire world … but then decided we only need to look at a few days worth of data, because the experts who said we need to collect data for a year are now changing their minds …’ Right! The v-safe was designed to look at a year of data … but we decided to only look at seven days. And there is this bridge in Brooklyn I have for sale …
Also from the court’s decision (under Judge Matthew Kacsmaryk): “If “some scientists” - sponsored or platformed by Defendants - “have chosen to use” only the first week or two of data to report their vaccine is safe and effective, then other scientists should be permitted to access the data to “pierce the veil of administrative secrecy.”...
One study reported that 0.8% to 1.1% of users reported needing medical care according to the check-box data … However, when the raw data was released pursuant to separate FOIA litigation, it showed some 7.7% of V-safe users reported needing medical care and an additional 25% missing school or work or unable to perform normal activities …
Any concerning symptoms would necessarily be restricted to only the free-text responses, to date unexamined …”
Yet to Come:
What does the first data dump (some 390,000 entries) show? That’s a great question to which I have no answer as of yet. It is going to take some considerable work to sort through free text data and collate it into a meaningful picture of what is happening due to the shots. And we won’t have all the data for a year (if we are lucky). So for now, I am only going to share some preliminary findings and not trends from what the CDC has called: “the most intensive safety monitoring effort in U.S. history” (that we’d rather you not see). If interested, ICAN has made their data available to all comers, here: https://icandecide.org/v-safe-data/
So now we await investigative assessment of what the free-text data shows and what it means. This may take some time to become meaningful. As you can imagine (and I have perused the data) - much of it is simply people emoting, or mentioning things that are not significant, or chatting with the powers-that-be, or using non-standard language that makes searching difficult. Free text is by nature hard to sort. And we only have the very first ‘drop.’ So don’t expect too much too quickly - but I will be keeping an eye on this over the coming year as more comes out.
One preliminary report (from the dogged researchers at Daily Clout) shows how many times the word tachycardia (or similar) showed up in this initial release. They note 1585 instances which can be seen at the following link. Note that not all recipients would be familiar with the medical term tachycardia (rapidly beating heart) … so there could be others not seen.
https://dailyclout.io/v-safe-voices-tachycardia-post-covid-vaccination/
Elsewhere:
In other (but related) news, you may have heard of a recently released CDC and HHS sponsored study (do you have questions already?) that purportedly shows that adverse events from the mRNA shots are real but “exceedingly rare.” How reassuring. This study, released on February 12th, was done by the New Zealand based “Global COVID vaccine safety project.”
99 million shot recipients were included in this massive study. I will report some of the study’s findings and you tell me if you think that the adverse events are barely worth noting, as the authors suggest.
The authors report that their study of results from 10 sites internationally (why ten, which ten?) showed that indeed there were instances of myocarditis, pericarditis, Guillain-Barre’ syndrome and cerebral venous sinus thrombosis (CVST). They only looked at events that were reported in the first 42 days. They acknowledge difficulties in analysis due to the lack of standardized reporting and incomplete reporting for certain regions (including the US).
Some observers have noted that the term ‘rare’ occurrences was never defined in terms of what the authors believed ‘rare’ to mean. Also, the authors “pre-identified” the potential complications that they were looking for (neurological, cardiovascular and hematological) while ignoring any other potential issues/adverse events. Again, why?
Here are some findings comparing rates of certain adverse events to background or expected rates:
Guillain-Barre’ - 2.49X greater post first AstraZeneca shot.
CVST - 3.23X greater post first AstraZeneca shot.
Myocarditis - 6.1X higher after second Moderna shot.
Pericarditis - 6.91X after third AstraZeneca.
Some observers also note that if you only look at higher risk groups (younger males and myocarditis), the risk goes way up from these reported numbers and makes these groups even more at risk for significant adverse events of importance.
The numbers go on, but the point is that even in this CDC permitted study, there appears to be rather significant risk associated with these shots … and the CDC never allows the worst to be shown as I have demonstrated in the past.
The authors of this report even state that there should be further investigation “to confirm associations and assess clinical significance.”
Here’s one way to look at this as described by a commentator who goes by the moniker: ‘the ethical skeptic:’ “...when this Bloomberg article says ‘small increases’, it means small relative to the 99M sample base (i.e. Prevalence Increase) … But in terms of Deviation from Trend (how it is really done), we are looking at 20-70% increases in occurrence (4 to 25 sigma) across MULTIPLE death causes (40 or more). It’s OK, they only stole 0.5% of your money from 40 of your investment investments/assets. So you are fine going forward.”
The authors of this report even state that there should be further investigation “to confirm.”
I might also note that several of the researchers in this study have a history of being on the payroll of companies like Pfizer, Gilead Sciences, AbbVie and GlaxoSmithKline. So there’s that. Funding for the study came from the likes of the CDC, the New Zealand Ministry of Health and the Canadian Institute of Health. All of these entities are heavily tied to the promotion of the shots and would stand to lose if the shots proved anything but “safe and effective.”
Does this look like a case of ‘limited hangout’ once again? ‘Yes, there are adverse events. We can no longer deny that. But if we frame them a certain way, maybe you won’t notice that they are all risk and no reward.’ Remember that a safe drug would compare favorably to a placebo. These most definitely do not.
There is much going on these days in the mounting pushback to the official narratives we were fed. Momentum is building against the lies we have been told. There is much more to come.
Hey, Siri! Nice work!
In health,
DocofLastResort